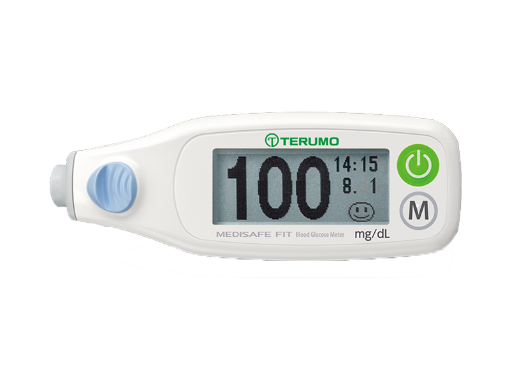
 Medisafe FIT®
Medisafe FIT®
Blood Glucose Meter
Description
The Medisafe Fit blood glucose meter is a high quality in vitro diagnostic medical device, which combines reliability and ease of use.
The design of the meter and the single-packed test TIPs are pleasantly different, meeting the patient’s needs.
Characteristics
Helpful guidance
The display shows helpful on-screen instructions and patient guidance:
-
- Easy to navigate with text and illustrations
- Easy to read with universal font
- Easy to understand error messages and suggestions to correct the error
 Patient support
Patient support
-
- Displays a smiley for blood glucose levels in the target range
- Alerts when blood glucose levels are outside the target range
 Post-Meal marking
Post-Meal marking
Post-Meal marking helps patient by:
-
- Managing glucose levels related to meals
- Adjusting target ranges related to pre- and post-meal values

Ergonomic FIT-curve design
-
- Fits to a variety of patient’s holding styles
- Allows to measure when placed on the table

Exceptional Test TIP
-
- Individually packed Test TIPs are protected from environmental influence, like humidity and dirt
- One-way Test TIP ensures correct attachment every time
- The nozzle-like tip permits easy blood sampling
- Clean disposal with the ejector lever
- No coding
Simple data transfer
 Wireless data transfer via Near Field Communication (NFC) technology. Placing the meter on top of the NFC reader unit allows records to be transferred directly to a computer.
Wireless data transfer via Near Field Communication (NFC) technology. Placing the meter on top of the NFC reader unit allows records to be transferred directly to a computer.
(Requires an NFC Reader)
DIABASS® is a registered trademark of mediaspects GmbH.
Technology that provides high accuracy
The Dual RS™ measurement technology with twin-beam optical sensors provides very accurate and reliable results.
- Reliable within hematocrit value 20-60%
- Not affected by dissolved oxygen or maltose
- Recognition of insufficient blood sample
ISO 15197:2015 compliance1
Meets ISO 15197:20151 accuracy requirements in the laboratory and at the user performance.2

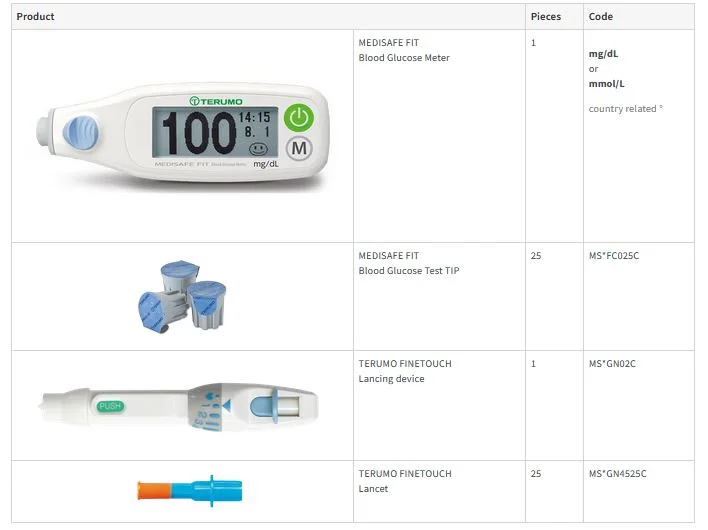
Item specifications
MEDISAFE FIT Blood Glucose Monitoring System
References
1 ISO (International Organization for Standardization). EN ISO 15197:2015 In vitro diagnostic test systems – Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197:2013), Geneva, Switzerland, 2015.
2 Freckmann G. et al. Analytical and User Performance Evaluation of a Blood Glucose Monitoring System following ISO 15197:2013, FREC1454D – Poster Session; 6th November 2014; Diabetes Technology Meeting 2014, Bethesda, Maryland, USA, 6th – 8th November 2014.




